Multiple Sclerosis
MULTIPLE SCLEROSIS (MS)
What is MS?
MS is an unpredictable autoimmune condition affecting the brain and spinal cord. The body attacks the protective sheath (myelin) that covers the nerve fibers. When the myelin sheath is damaged or destroyed, this can severely slow the process of transmitting information from the brain to the body. It can range from being benign
What are the signs and symptoms?
- Numbness or weakness in one or more limbs that typically occurs on one side of your body at a time, or the legs and trunk
- Electric-shock sensations that occur with certain neck movements
- Tremor, lack of coordination or unsteady gait
- Vision – partial or complete loss of vision (usually in one eye at a time), prolonged double vision, blurry vision
- Slurred speech
- Fatigue
- Dizziness
- Problems with sexual, bowel and bladder function
How is MS diagnosed?
There are no specific tests to diagnose MS but the doctor may rule out other conditions that have similar signs and symptoms. A series of tests and screening exams, medical history and thorough assessment helps in the diagnosis.
Role of Functional Medicine in MS
Functional Medicine is directed towards the underlying causes of the patient’s symptoms by addressing inflammation issues. The main goal is to enhance the body’s ability to care for itself and to support the immune system as a whole, reduce symptoms or delay the development of the disease.
The treatment plans vary per patient and their specific symptoms. Common approach would include nutritional assessment and revision of the patient’s diet, detect digestive problems and treat with FMT, eliminating possible toxins in the blood through antioxidants treatment, identifying infections, and appropriate physiotherapy activities.
- Published in Neurological Rehabilitation Center, Services
IN VITRO FERTILIZATION (IVF)
What is IVF?
IVF is considered an effective Assisted Reproductive Technology. It is a procedure used to help with fertility problems by conceiving a child through fertilization in the laboratory. Fertilization is done by extracting mature eggs from ovaries and a sperm sample and manually uniting both in a laboratory dish. Once eggs are fertilized fully, it is transferred to the uterus.
Functional Medicine’s Role in IVF
At Better Being Hospital, our Functional Medicine practice aims to support the extensive and expensive efforts of IVF. Nutritional, environmental, lifestyle, physiologic factors and many others are taken into consideration and evaluated to determine if there are any aspects that would have an impact on the chances of IVF’s success. In cases where patients experience implantation failure, Functional Medicine determines the contributing issues such as autoimmunity, inflammation or deficiencies and formulates a supportive treatment plan to improve the patient’s overall health.
- Published in Infertile Rehabilitation Center, Services
Hormone Imbalance
HORMONAL IMBALANCE
What is Hormonal Imbalance?
Hormonal is the excess or lack of certain hormones. Hormones are the body’s chemical
messenger. These are produced in endocrine glands and travel around through the blood to
regulate activities of cells and organs. It controls major body processes like metabolism and
reproduction. Small changes in the hormones can have serious effects on the bodily functions.
What are the types of Hormones?
The most common hormones and their functions are the following:
- Thyroid— regulates physical growth and development, weight, energy levels, internal temperature, and many more.
- Estrogen— development of female secondary sexual characteristics including breasts, endometrium, and regulation of the menstrual cycle. In males estrogen helps in maturation of the sperm and maintenance of a healthy libido.
- Progesterone— responsible for the regulation of uterine function during menstruation and plays a role in reproduction and maintaining the early stages of pregnancy.
- Testosterone— responsible for the development of male sexual characteristics, sperm production, sex drive, bone mass, fat distribution, muscle size and strength and many others.
What causes Hormonal Imbalances?
There are many known causes and conditions that impact the endocrine glands. Some are lifestyle habits and environmental factors.
- chronic or extreme stress
- Type 1 and Type 2 diabetes, hyperglycemia or hypoglycemia
- hypothyroidism or hyperthyroidism
- poor diet and nutrition
- obesity or anorexia
- hormonal replacement or birth control medications
- abuse of anabolic steroid medications
- pituitary tumors
- Diseases such as Cushing’s syndrome, Addison’s disease
- benign or malignant tumors and cysts affecting the endocrine glands
- injury to endocrine glands
- severe allergic reactions or infections
- chemotherapy and radiation therapy
- iodine deficiency (goiters)
- exposure to toxins, pollutants, and endocrine disrupting chemicals, including pesticides and herbicides
Common signs and symptoms
The symptoms depend on the glands and hormones affected, however there are a few
common symptoms that are indicative of a hormonal imbalance in general.
- unexplained weight gain or weight loss
- unexplained or excessive sweating
- difficulty sleeping
- changes in sensitivity to cold and heat
- very dry skin or skin rashes
- changes in blood pressure and heart rate
- brittle or weak bones
- changes in blood sugar concentration
- irritability and anxiety
- unexplained and long-term fatigue
- depression
- frequent headaches
- changes in appetite
- reduced sex drive
- thinning, brittle hair
- infertility
Role of Functional Medicine
Functional Medicine seeks to identify and treat the underlying cause, create and support
the balance of hormones to improve one’s health and reduce symptoms. It involves a careful
investigation and interpretation of various tests to detect markers for inflammation, oxidative
stress, metabolism, nutrient levels, genetic testing and chronic infections. Certain modifications in
diet and lifestyle may be advised as well as nutritional supplements and other beneficial therapies
such as Fecal Microbiota Transplantation, Antioxidants and stem cell therapy depending on the
cause of imbalance.
- Published in Functional Medicine Clinic, Services
FMT, Fecal microbiota transplant
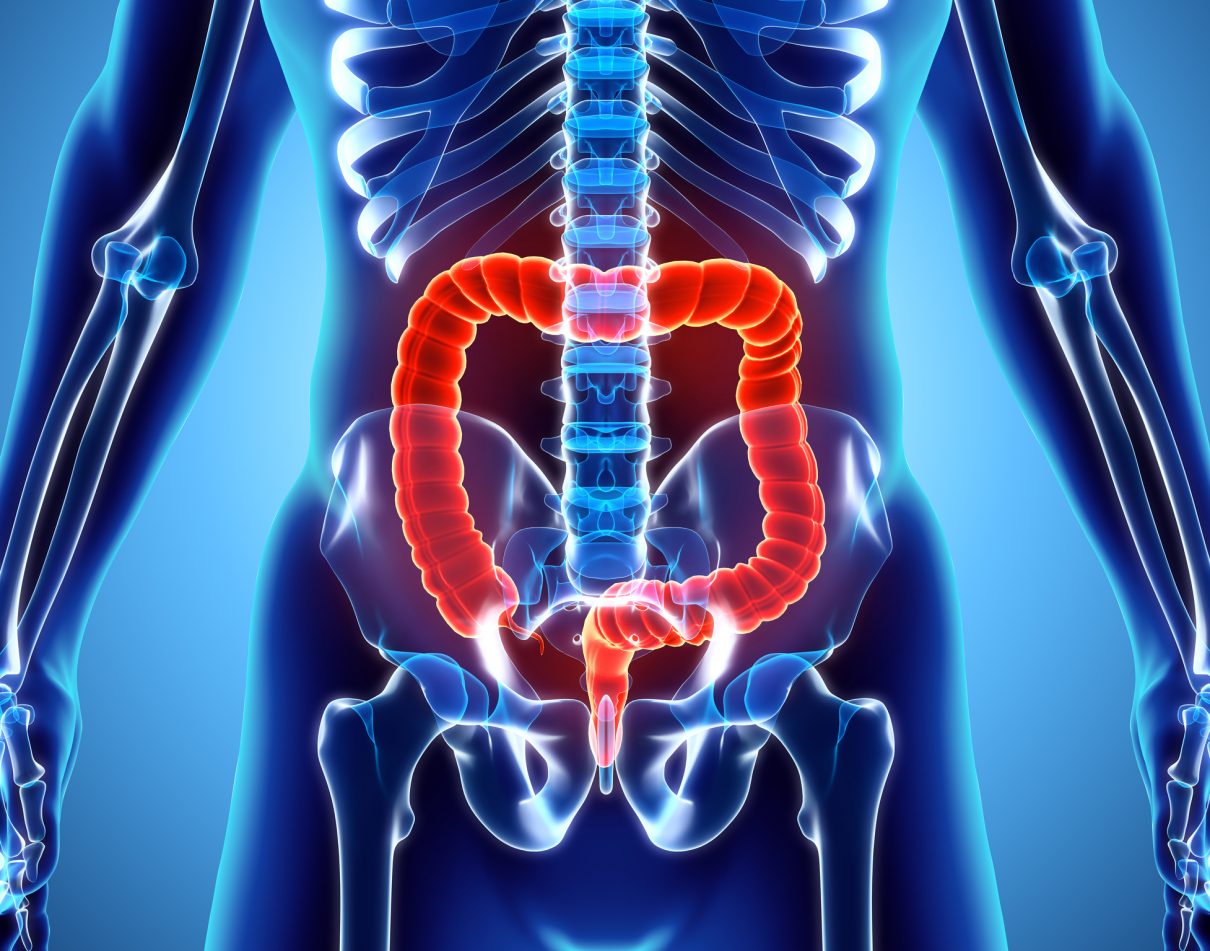
FECAL MICROBIOTA TRANSPLANT (FMT)
What is FMT?
FMT is the process of transplanting intestinal microbiota (intestinal flora) of a healthy individual to a recipient (patient). The preparation is done from a meticulously screened and healthy stool donor. FMT promotes the restoration of the patient’s intestinal microbiota by introducing healthy bacteria. The goal of FMT is to remove the pathogenic infection and rebalance the gut flora in order to alleviate or stop the symptoms.
How is FMT done?
FMT can be administered orally by capsules or through enema – inserting a tube into the patient’s rectum and infusing the liquid solution into the colon.
Who are candidates for FMT?
People with certain chronic illnesses usually take antibiotics for long periods. Taking antibiotics repetitively causes a disruption in the normal ecosystem of protective bacterial species (intestinal flora) in the intestines. These bacteria are actually needed by the body, especially for the gut to function properly. Once the normal intestinal flora is damaged and not enough to compete with harmful pathogens in the gut, these pathogens reside in the gut and produce toxins that cause the patient’s symptoms.
Conditions that can benefit from FMT:
- Autoimmune diseases
- Neurological conditions
- Metabolic syndromes such as inflammatory bowel diseases (such as ulcerative colitis and Crohn’s disease etc.)
- Clostridium difficile infection
- Amyotrophic lateral sclerosis (ALS)
- Multiple sclerosis
- Autism
- Alzheimer’s syndrome
- Parkinson’s disease
- Type 2 diabetes
- Obesity
- Numerous gastrointestinal conditions
- Published in Gastrointestinal Rehabilitation Center, Services
CEREBRAL PALSY (CP)
CEREBRAL PALSY (CP)
What is CP?
Cerebral Palsy is a combination of problems that affects ones muscle tone, movement and motor skills. A person with CP does not have coordination of movement. It can affect other basic but essential body functions such as breathing, bladder and bowel control, eating and talking. It often stems from brain damage that occurs before or during birth or from 3-5 years of a child’s life. Conditions like vision, hearing and learning problems may arise from CP. However, it does not progress or get worse over time.
What causes CP?
There is no definite cause. It happens during the brain’s development. It may be due to one factor or a combination of many issues. Some known factors that contribute to CP are:
- infections during pregnancy
- brain damage due to several causes such as injuries or meningitis
- complications in premature birth (poor blood flow to the brain)
- untreated jaundice
- mother’s medical problems during pregnancy
What are the types of CP?
- Spastic Cerebral Palsy — most common. Decreased muscle tone causes stiffness and jerky movements.
- Dyskinetic – characterized by uncontrolled movements that can be twisting, repetitive, slow, or unpredictable. Can be very severe.
- Ataxic – characterized by shaky movements that affects balance, coordination, posture positioning and even eye movements.
Role of Functional Medicine in CP
The goal in CP patients is to assist in improving brain and neurological function and improve symptoms. Integrative and customized neuro-regenerative programs are designed for each patient depending on their condition. Adjuvant therapies can also be identified and combined with our physiotherapy programs such as Robotics Training, G-Factor and Transcranial Magnetic Stimulation.
- Published in Neurological Rehabilitation Center, Services
CANCER
What is cancer?
Cancer is a collection of diseases which starts when cells start to grow uncontrollably. Normally, human cells grow and divide to form new cells as needed by the body while old or damaged cells die. With cancer cells, there is an abnormal production and elimination of cells. These cells may grow and form into a tumor which if malignant, can spread and grow to other parts of the body.
Signs and symptoms of cancer will depend on the body part that is affected.
One’s predisposition to cancer may or may not be prevented. Depending on the type and period of detection, cancer can be treated.
Types of cancer
There are many types of cancer. Some examples are:
- Carcinomas – most common type of cancer which usually forms solid tumors. Examples are prostate cancer, breast cancer, lung cancer, and colorectal cancer.
- Sarcomas – usually developes in the tissues that support and connect the body such as fat, muscles, nerves, tendons, joints, blood vessels, lymph vessels, cartilage, or bone.
- Leukemias – more popular as cancer of the blood.
- Lymphomas – cancer that begins in the lymphatic system.
Role of Functional Medicine in Cancer
Functional Medicine focuses on the preventive and post-therapeutic condition of patients who are treated conventionally. Conventional approach to cancer would target the tumor or the affected area by popular means such as surgery, chemotherapy, radiation, and others. While Functional Medicine respects the therapeutic means of conventional medicine, it digs deeper and investigates how the body became susceptible to this illness, such as determining the patient’s risk factors, their stress levels, dietary habits, other health conditions that compromised their immune system. To get a better understanding of the tumor environment, objective testing is done with examination of lab values to help identify biomarkers of chronic inflammation, oxidative stress, glucose metabolism, insulin resistance and hormonal issues.
Once the doctor has taken all these in consideration, diet and lifestyle modification, nutrition supplementation, improving and strengthening immunity. Antioxidant therapy, acupuncture and hyperbaric oxygen therapy may be incorporated as well.
- Published in Cancer Rehabilitation Center, Services
SCI
Introduction: According to the National Spinal Cord Injury Association, as many as 430,It was estimated that 17,000 new SCIs occur in the U.S. Spinal cord injuries can be caused by many factors, such as road accidents (45%), falls from high places (20%), sports injuries (15%), violence (15%), and other activities (5%) BUT most of these are caused by trauma to the vertebral column, thereby affecting the spinal cord’s ability to send and receive messages from the brain to the body’s systems that control sensory, motor and autonomic function below the level of injury.
According to the Centers for Diseases Control and Prevention (CDC), SCI costs the nation an estimated 10 billion USD each year.
. Osteoporosis and spinal tumors can also cause spinal fractures. These conditions account for 80% of spinal fractures in patients between the ages of 18-25 years. As such, males are approximately four times more likely than females to experience spinal fractures.
Sign and Symptom: Symptoms will depend on the severity and location of the spinal cord injury. Individuals who present with spinal cord injuries may experience neck pain, back pain, or pain in other affected areas, as well as lack of sensation (numbness), muscle tension, fatigue, and incontinence. Some people can also experience paralysis, which indicates an injury to both the nervous system and spinal cord. A complete SCI produces total loss of all motor and sensory function below the level of injury. Nearly 50% of all SCIs are complete. Both sides of the body are equally affected. Even with a complete SCI, the spinal cord is rarely cut or transected. More commonly, loss of function is caused by a contusion or bruise to the spinal cord or by compromise of blood flow to the injured part of the spinal cord.
In an incomplete SCI, some function remains below the primary level of the injury. A person with an incomplete injury may be able to move one arm or leg more than the other or may have more functioning on one side of the body than the other.
SCIs are graded according to the American Spinal Injury Association (ASIA) grading scale, which describes the severity of the injury. The scale is graded with letters:
- ASIA A: injury is complete spinal cord injury with no sensory or motor function preserved.
- ASIA B: a sensory incomplete injury with complete motor function loss.
- ASIA C: a motor incomplete injury, where there is some movement, but less than half the muscle groups are anti-gravity (can lift up against the force of gravity with a full range of motion).
- ASIA D: a motor incomplete injury with more than half of the muscle groups are anti-gravity.
- ASIA E: normal.
The more severe the injury, the less likely a recovery will occur.
Spinal concussions can also occur. These can be complete or incomplete, but spinal cord dysfunction is transient, generally resolving within one or two days. Football players are especially susceptible to spinal concussions and spinal cord contusions. The latter may produce neurological symptoms, including numbness, tingling, electric shock-like sensations and burning in the extremities.
Open or penetrating injuries to the spine and spinal cord, especially those caused by firearms, may present somewhat different challenges. Most gunshot wounds to the spine are stable; i.e., they do not carry as much risk of excessive and potentially dangerous motion of the injured parts of the spine. Depending upon the anatomy of the injury, the patient may need to be immobilized with a collar or brace for several weeks or months so that the parts of the spine fractured by the bullet heals. In most cases, surgery to remove the bullet does not yield much benefit and may create additional risks, including infection, cerebrospinal fluid leak and bleeding. However, occasional cases of gunshot wounds to the spine may require surgical decompression and/or fusion in an attempt to optimize outcome.
Diagnosis
Patients who have been involved in serious accidents, and present with the symptoms listed above, will need to be cautious with their range of movement, and will also require extra physical support such as wearing a back brace. After conducting a comprehensive physical examination of the patient, the doctor will issue a suitable diagnosis and treatment plan, such as a CT or MRI scan. An MRI scan is usually required in cases of suspected nervous system or spinal cord injury.
Treatment: Treatment of SCI begins before the patient is admitted to the hospital. Paramedics or other emergency medical services personnel carefully immobilize the entire spine at the scene of the accident. In the emergency department, this immobilization is continued while more immediate life-threatening problems are identified and addressed. If the patient must undergo emergency surgery because of trauma to the abdomen, chest or another area, immobilization and alignment of the spine are maintained during the operation.
Non-Surgical Treatments
If a patient has a SCI, he or she will usually be admitted to an intensive care unit (ICU). For many injuries of the cervical spine, traction may be indicated to help bring the spine into proper alignment. Standard ICU care, including maintaining a stable blood pressure, monitoring cardiovascular function, ensuring adequate ventilation and lung function and preventing and promptly treating infection and other complications, is essential so that SCI patients can achieve the best possible outcome.
Surgery
Occasionally, a surgeon may wish to take a patient to the operating room immediately if the spinal cord appears to be compressed by a herniated disc, blood clot or other lesion. This is most commonly done for patients with an incomplete SCI or with progressive neurological deterioration. Even if surgery cannot reverse damage to the spinal cord, surgery may be needed to stabilize the spine to prevent future pain or deformity. The surgeon will decide which procedure will provide the greatest benefit to the patient
What Makes Our Stem Cell Treatment for Spinal Cord Injury Effective ?
Since 2005, we have been developing comprehensive stem cell treatment for spinal cord injury protocols to overcome the limitations of conventional therapies. In our protocols, stem cells are combined with specialized therapies for spinal cord injury that not only focus on helping the patient to cope with their symptoms, but also treat the root cause of the condition by promoting the healing of the original injury. We believe that our comprehensive stem cell therapy for spinal cord injury gives the best chances of improvements, allowing for a better quality of life. Read more
Optionally and in addition to our stem cell treatment for spinal cord injury protocol, we are providing a cutting edge epidural stimulation technology* that helps neurological signals to bypass the injury site in the spinal cord, helping to regain function below the injury.
What potential improvements after therapy?
The purpose of stem cell therapy is to promote the healing of the original injury in order to restore neurological function. Thus, various kinds of improvement are possible after our treatment and our past patients have experienced the following*:
- Improved motor function
- Increased sensation
- Decreased spasticity
- Decreased neuropathic pain
- Improve bladder & bowel function
- Increase sweating function
- Pain relief
- Published in Services
Introduction Spinal muscular atrophy (SMA) is a genetic disease affecting the part of the nervous system that controls voluntary muscle movement.
Most of the nerve cells that control muscles are located in the spinal cord, which accounts for the word spinal in the name of the disease. SMA is muscular because its primary effect is on muscles, which don’t receive signals from these nerve cells. Atrophy is the medical term for getting smaller, which is what generally happens to muscles when they’re not active.
SMA involves the loss of nerve cells called motor neurons in the spinal cord and is classified as a motor neuron disease.
In the most common form of SMA (chromosome 5 SMA, or SMN-related SMA), there is wide variability in age of onset, symptoms and rate of progression. In order to account for these differences, the chromosome 5 SMA often is classified into types 1 through 4.
The age at which SMA symptoms begin roughly correlates with the degree to which motor function is affected: The earlier the age of onset, the greater the impact on motor function. Children who display symptoms at birth or in infancy typically have the lowest level of functioning (type 1). SMA onset in children (types 2 and 3), teens or adults (type 4) generally correlates with increasingly higher levels of motor function.
Chromosome 5 SMA is caused by a deficiency of a motor neuron protein called SMN, for “survival of motor neuron.” This protein, as its name implies, seems to be necessary for normal motor neuron function. Its deficiency is caused by genetic flaws (mutations) on chromosome 5 in a gene called SMN1. Neighboring SMN2 genes can in part compensate for nonfunctional SMN1 genes.
The primary symptom of chromosome 5-related (SMN-related) SMA is weakness of the voluntary muscles. The muscles most affected are those closest to the center of the body, such as those of the shoulders, hips, thighs and upper back. Special complications occur if the muscles used for breathing and swallowing are affected, resulting in abnormalities in these functions. If the muscles of the back weaken, spinal curvatures can develop.
There’s a great deal of variation in the age of onset and level of motor function achieved in chromosome 5-related SMA. These are roughly correlated with how much functional SMN protein is present in the motor neurons, which in turn is correlated with how many SMN2 genes a person has.
Sensory, mental and emotional functioning are entirely normal in chromosome-5 SMA.
Some forms of SMA are not linked to chromosome 5 or SMN deficiency. These forms vary greatly in severity and in the muscles most affected. While most forms, like the chromosome 5-related form, affect mostly the proximal muscles, other forms exist that affect mostly the distal muscles (those farther away from the body’s center) — at least in the beginning.
Causes
SMA types 0, 1, 2, 3 and 4 are inherited as autosomal recessive genetic disorders and are associated with abnormalities (mutations) in the SMN1 and SMA2 genes on chromosome 5 at chromosomal locus 5q11-q13. SMA1 is thought to be the primary disease-causing gene. Approximately 95-98% of affected individuals have deletions in the SMA1 gene and 2-5% have specific mutations in the SMA1 gene that result in a decreased production of the SMN protein. When three or more copies of the SMA2 gene are also present, the disease may be milder.
Genetic diseases are determined by the combination of genes for a particular trait that are on the chromosomes received from the father and the mother.
Recessive genetic disorders occur when an individual inherits the same abnormal gene for the same trait from each parent. If an individual receives one normal gene and one gene for the disease, the person will be a carrier for the disease, but usually will not show symptoms. The risk for two carrier parents to both pass the defective gene and, therefore, have an affected child is 25% with each pregnancy. The risk to have a child who is a carrier like the parents is 50% with each pregnancy. The chance for a child to receive normal genes from both parents and be genetically normal for that particular trait is 25%. The risk is the same for males and females.
Biological or surrogate markers for SMA are under development. These potential surrogate markers studied to date include:
1) Measuring the amount and ratio of full-length and truncated SMN2 RNA transcripts as well as the amount of SMN protein from white blood cells or fibroblast culture.
2) Counting motor units (Motor Unit Number Estimation, or MUNE) which have shown correlation with the SMN2 copy number, age, and function.
3) Quantitative ultrasound in assessing muscle changes in patients with SMA.
4) Electrical impedance myography.
5) The compound action potential determined by EMG in children with SMA. The authors point out that MUNE is difficult and not as reliable as initially thought. A longitudinally study of the surrogate markers will be necessary before any particular one can be used in an intervention study.
Subdivisions of Spinal Muscular Atrophy
- SMA type 0
- SMA type 1
- SMA type 2
- SMA type 3
- SMA type 4
Sign and Symptoms
SMA type 0 is the most severe form of the disease and is characterized by decreased fetal movement, joint abnormalities, difficulty swallowing and respiratory failure.
SMA type 1 is the most common type of SMA and is also a severe form of the disease. Infants with SMA type 1 experience severe weakness before 6 months of age and never sit independently. Muscle weakness, lack of motor development and poor muscle tone are the major clinical manifestations of SMA type I. Infants with the gravest prognosis have problems sucking or swallowing. Some show abdominal breathing in the first few months of life. Muscle weakness occurs on both sides of the body and the ocular muscles are not affected. A twitching of the tongue is often seen. Intelligence is normal. Most affected children die before two years of age but survival may be dependent on the degree of respiratory function. For more information about SMA type 1, chose “Werdnig Hoffman” disease as your search term in the Rare Disease Database.
The onset of weakness in SMA type 2 patients is usually between 6 and 12 months. Affected children are able to sit independently early in development but are unable to walk even 10 feet independently. A trembling (tremor) of the fingers is almost always seen in SMA type 2. Approximately 70% of those affected do not have deep tendon reflexes. Those affected with SMA type 2 are usually not able to sit independently by the mid-teens or later.
Patients with SMA type 3 (Kugelberg-Welander syndrome) learn to walk but fall frequently and have trouble walking up and down stairs at 2-3 years of age. The legs are more severely affected than the arms. The long-term prognosis depends on the degree of motor function attained as a child. For more information about SMA3 chose “Kugelberg Welander syndrome” as your search term in the Rare Disease Database.
The onset of muscle weakness for those with SMA type 4 is after age 10 years; these patients usually are ambulatory until age 60 years.
Complications of SMA include scoliosis, joint contractures, pneumonia and metabolic abnormalities such as severe metabolic acidosis and dicarboxylic aciduria.
Diagnosis
The diagnosis of SMA is suspected when symptoms are present and the diagnosis can be confirmed with molecular genetic testing. Molecular genetic testing is used to determine if a mutation is present in the SMN1 gene. SMA types 0, 1, 2, 3 and 4 are caused by a partial or complete loss of the SMN1 gene and about 95% of those affected will show a deletion of both copies of a specific portion (exon 7 or exon 8) of the gene. About 5% of those affected will show a deletion of exon 7 in one copy of the SMN1 gene and a different mutation in the other copy of the SMN1 gene. Molecular genetic testing can also be used to determine the number of copies of the SMN2 gene.
Prior to the availability of molecular testing, neurophysiologic studies and muscle biopsy were used for diagnosis, but these tests are no longer necessary unless SMN gene testing is normal.
Carrier testing for SMA is available using a molecular genetic test in which the number of copies of the SMN1 gene is determined.
Molecular genetic testing for the VAPB gene is available to diagnose Finkel type SMA.
Care for individuals with SMA is symptomatic and includes physical therapy, occupational therapy, monitoring of respiratory function and nutritional status, orthotics and adaptive equipment. Respiratory support for SMA1 using a breathing machine called BiPAP (bi-level positive airway pressure) has been shown to increase comfort and life expectancy in some affected children.
Genetic counseling is recommended for affected individuals and their families
Treatment
The management of children with spinal muscular atrophy starts with the diagnosis and classification into 1 of the 5 categories. Health issues specific to spinal muscular atrophy are as follows:
Pulmonary management: Children with SMA1 can survive beyond 2 years of age when offered tracheostomy or noninvasive respiratory support.
An intermittent positive-pressure breathing device (mechanical in-exsufflator) has proven effective.
Nutrition: Bulbar dysfunction is universal in SMA1 patients. Early gastrostomy should be considered as part of the management of such patients. The bulbar dysfunction eventually becomes a serious problem for spinal muscular atrophy II patients and only very late in the course of disease for spinal muscular atrophy III patients.
Scoliosis: Scoliosis is a major problem in most SMA2 patients and in half of SMA3 patients.
The vertical expandable prosthetic titanium rib (VEPTR) was approved by the FDA in 2004 as a treatment for thoracic insufficiency syndrome (TIS) in pediatric patients. TIS is a congenital condition where severe deformities of the chest, spine, and ribs prevent normal breathing and lung development. The VEPTR is an implanted, expandable device that helps straighten the spine and separate ribs so that the lungs can grow and fill with enough air to breathe. The length of the device can be adjusted as the patient grows. The titanium rib was developed at the University of Texas Health Science Center in San Antonio. It is manufactured by Synthes Spine Co.
Hip dislocation: Hip dislocation is another orthopedic concern in patients with spinal muscular atrophy. If the hip dislocation is asymptomatic, surgery is not indicated.
Behavior issues: Compared to siblings and normal controls, patients with spinal muscular atrophy were quite well adjusted. Concern was, however, raised about unaffected siblings, who had a 2 to 3-fold higher rate behavioral problems than normal children.
Sleep disorders: Sleep-disordered breathing may develop prior to respiratory failure. Night-time use of continuous positive airway pressure with a nasal mask may be helpful.
In 2017, Spinraza (nusinersen) was FDA approved as the first drug to treat children and adults with SMA. Spinraza is manufactured by Biogen.
How Functional medicine can help this condition?
What Makes our Stem Cell Treatment for SMA Effective?
Since 2005, we have been developing comprehensive protocols regarding stem cell treatment for Spinal Muscular Atrophy (SMA) to overcome the limitations of conventional therapies. In our protocols, stem cells are combined with specialized therapies for spinal muscular atrophy that not only focus on helping the patient to cope with their symptoms, but also treat the direct cause of the symptoms by promoting the healing of the injury in the spinal cord. We believe that our comprehensive stem cell treatment for spinal muscular atrophy gives our patients the best chances of improvements, allowing for a better quality of life. Different types of spinal muscular atrophy can be considered for treatment such as SMA type 1, SMA type 2, SMA type 3 (Kugelberg-Welander syndrome) and more.
What potential improvements after the treatment?
The purpose of our treatment for spinal muscular atrophy is to promote the healing of the injury in the spinal cord in order to restore neurological function. Thus, various kinds of improvement are possible after our treatment and our past patients have experienced the following*:
- Improved range of movements
- Increased muscle mass
- Increased muscle strength
- Better coordination
- Improved balance
- Improved development
Decreased muscle rigidity
- Published in Services
Spinal bifida
Introduction: Spina bifida is a condition that affects the spine and is usually apparent at birth. It is a type of neural tube defect (NTD).
Spina bifida can happen anywhere along the spine if the neural tube does not close all the way. When the neural tube doesn’t close all the way, the backbone that protects the spinal cord doesn’t form and close as it should. This often results in damage to the spinal cord and nerves.
Spina bifida might cause physical and intellectual disabilities that range from mild to severe. The severity depends on:
- The size and location of the opening in the spine.
Whether part of the spinal cord and nerves are affected.
Causes of spina bifida
The cause of spina bifida is unknown, but a number of factors can increase the risk of a baby developing the condition.
These include:
- low folic acid intake during pregnancy
- having a family history of spina bifida
- medication – taking certain medications during pregnancy has been linked to an increased risk of having a baby with spina bifida
Sign and Symptom: Types of Spina Bifida
The three most common types of spina bifida are Myelomeningocele (sounds like: my-low-ma-nin-jo-seal; hear how “myelomeningocele” sounds
When people talk about spina bifida, most often they are referring to myelomeningocele. Myelomeningocele is the most serious type of spina bifida. With this condition, a sac of fluid comes through an opening in the baby’s back. Part of the spinal cord and nerves are in this sac and are damaged. This type of spina bifida causes moderate to severe disabilities, such as problems affecting how the person goes to the bathroom, loss of feeling in the person’s legs or feet, and not being able to move the legs.
Meningocele (sounds like: ma-nin-jo-seal; hear how “meningocele” sounds
Another type of spina bifida is meningocele. With meningocele a sac of fluid comes through an opening in the baby’s back. But, the spinal cord is not in this sac. There is usually little or no nerve damage. This type of spina bifida can cause minor disabilities.
Spina Bifida Occulta (sounds like: o-cult-tuh; hear how “occulta” sounds
Spina bifida occulta is the mildest type of spina bifida. It is sometimes called “hidden” spina bifida. With it, there is a small gap in the spine, but no opening or sac on the back. The spinal cord and the nerves usually are normal. Many times, spina bifida occulta is not discovered until late childhood or adulthood. This type of spina bifida usually does not cause any disabilities.
Diagnosis: Diagnosing spina bifida
Most cases of spina bifida are detected during the mid-pregnancy anomaly scan, which is offered to all pregnant women between 18 and 21 weeks of pregnancy.
If tests confirm that your baby has spina bifida, the implications will be discussed with you.
This will include a discussion about the possible problems associated with the condition, the treatment and support your child may need if you decide to continue with the pregnancy, and what your options are regarding ending the pregnancy, if that’s your choice.
Tests after birth
Once the baby is born, a number of tests may be carried out to assess the severity of the condition and help decide which treatments are appropriate.
Tests may include:
- monitoring your child’s head growth and carrying out a brain scan, using an ultrasound scan, CT scan or MRI scan, to check for hydrocephalus (excess fluid on the brain)
- ultrasound scans of the bladder and kidneys to check whether your baby stores urine normally
- an assessment of your baby’s movements to check for paralysis
In most cases, surgery to repair the spine will be recommended soon after your baby is born.
Treating spina bifida
Treatments for the symptoms or conditions associated with spina bifida include:
- surgery soon after birth to close the opening in the spine and treat hydrocephalus
- therapies to help make day-to-day life easier and improve independence, such as physiotherapy and occupational therapy
- assistive devices and mobility equipment, such as a wheelchair, or walking aids
- treatments for bowel and urinary problems
With the right treatment and support, many children with spina bifida survive well into adulthood.
It can be a challenging condition to live with, but many adults with spina bifida are able to lead independent and fulfilling lives.
Read more about treating spina bifida
Treatment: Treatments for the symptoms or conditions associated with spina bifida include:
- surgery soon after birth to close the opening in the spine and treat hydrocephalus
- therapies to help make day-to-day life easier and improve independence, such as physiotherapy and occupational therapy
assistive devices and mobility equipment, such as a wheelchair, or walking aids
- treatments for bowel and urinary problems
With the right treatment and support, many children with spina bifida survive well into adulthood.
It can be a challenging condition to live with, but many adults with spina bifida are able to lead independent and fulfilling lives.
What Makes our Treatment for Spina Bifida Effective?
Since 2005, we have been developing comprehensive stem cell therapy protocols for Spina Bifida to overcome the limitations of conventional therapies. In our protocols, stem cells are combined with specialized therapies for spina bifida that not only focus on helping the patient to cope with their symptoms, but also treat the root cause of the condition by promoting the healing of the original spinal cord/brain injury. We believe that our comprehensive therapy approach for spina bifida gives our patients the best chances of improvements, allowing for a better quality of life.
What potential improvements after the treatment?
The purpose of the treatment is to promote healing of the original spinal cord/brain injury in order to restore neurological function. Thus, various kinds of improvement are possible after our treatment and our past patients have experienced the following*:
- Improved motor function
- Increased sensations
- Improved development
- Improved mental abilities
- Increased muscle strength
- Reduced epilepsy seizures
- Enhanced bladder & bowel function
- Published in Neurological Rehabilitation Center, Services
Osteoporosis
OSTEOPOROSIS
Introduction: Osteoporosis, which literally means porous bone, is a disease in which the density and quality of bone are reduced. As bones become more porous and fragile, the risk of fracture is greatly increased. The loss of bone occurs silently and progressively. Often there are no symptoms until the first fracture occurs.
Osteoporosis affects men and women of all races. But white and Asian women — especially older women who are past menopause — are at highest risk. Medications, healthy diet and weight-bearing exercise can help prevent bone loss or strengthen already weak bones.
As we age some of our bone cells begin to dissolve bone matrix (resorption), while new bone cells deposit osteoid (formation). This process is known as remodeling.
For people with osteoporosis, bone loss outpaces the growth of new bone. Bones become porous, brittle and prone to fracture. Around the world, 1 in 3 women and 1 in 5 men aged fifty years and over are at risk of an osteoporotic fracture. In fact, an osteoporotic fracture is estimated to occur every 3 seconds. The most common fractures associated with osteoporosis occur at the hip, spine and wrist. The likelihood of these fractures occurring, particularly at the hip and spine, increases with age in both women and men.
Of particular concern are vertebral (spinal) and hip fractures. Vertebral fractures can result in serious consequences, including loss of height, intense back pain and deformity (sometimes called Dowager’s Hump). A hip fracture often requires surgery and may result in loss of independence or death.
Cause
- The loss of estrogen due to menopause is the most common cause of osteoporosis. In fact, 25% of women older than 60 years old are found to have osteoporosis.
- Women who go through menopauseearly or those who have had their ovaries surgically removed before the age of 45 are at risk.
The aging process is a major factor because, by the age of 50, bones start thinning by 1-3% every year.
Unchangeable risks
Some risk factors for osteoporosis are out of your control, including:
- Your sex. Women are much more likely to develop osteoporosis than are men.
- Age. The older you get, the greater your risk of osteoporosis.
- Race. You’re at greatest risk of osteoporosis if you’re white or of Asian descent.
- Family history. Having a parent or sibling with osteoporosis puts you at greater risk, especially if your mother or father fractured a hip.
- Body frame size. Men and women who have small body frames tend to have a higher risk because they might have less bone mass to draw from as they age.
Hormone levels
Osteoporosis is more common in people who have too much or too little of certain hormones in their bodies. Examples include:
- Sex hormones. Lowered sex hormone levels tend to weaken bone. The reduction of estrogen levels in women at menopause is one of the strongest risk factors for developing osteoporosis.
Men have a gradual reduction in testosterone levels as they age. Treatments for prostate cancer that reduce testosterone levels in men and treatments for breast cancer that reduce estrogen levels in women are likely to accelerate bone loss.
- Thyroid problems. Too much thyroid hormone can cause bone loss. This can occur if your thyroid is overactive or if you take too much thyroid hormone medication to treat an underactive thyroid.
- Other glands. Osteoporosis has also been associated with overactive parathyroid and adrenal glands.
Dietary factors
Osteoporosis is more likely to occur in people who have:
- Low calcium intake. A lifelong lack of calcium plays a role in the development of osteoporosis. Low calcium intake contributes to diminished bone density, early bone loss and an increased risk of fractures.
- Eating disorders. Severely restricting food intake and being underweight weakens bone in both men and women.
- Gastrointestinal surgery. Surgery to reduce the size of your stomach or to remove part of the intestine limits the amount of surface area available to absorb nutrients, including calcium. These surgeries include those to help you lose weight and for other gastrointestinal disorders.
Steroids and other medications
Long-term use of oral or injected corticosteroid medications, such as prednisone and cortisone, interferes with the bone-rebuilding process. Osteoporosis has also been associated with medications used to combat or prevent:
- Seizures
- Gastric reflux
- Cancer
- Transplant rejection
Medical conditions
The risk of osteoporosis is higher in people who have certain medical problems, including:
- Celiac disease
- Inflammatory bowel disease
- Kidney or liver disease
- Cancer
- Lupus
- Multiple myeloma
- Rheumatoid arthritis
Lifestyle choices
Some bad habits can increase your risk of osteoporosis. Examples include:
- Sedentary lifestyle. People who spend a lot of time sitting have a higher risk of osteoporosis than do those who are more active. Any weight-bearing exercise and activities that promote balance and good posture are beneficial for your bones, but walking, running, jumping, dancing and weightlifting seem particularly helpful.
- Excessive alcohol consumption. Regular consumption of more than two alcoholic drinks a day increases your risk of osteoporosis.
- Tobacco use. The exact role tobacco plays in osteoporosis isn’t clear, but it has been shown that tobacco use contributes to weak bones.
Common risk factors
- A family history of osteoporosis
- European or Asian lineage
- Lack of vitamin D or calcium
- Regular consumption of alcohol or caffeine
- Smoking
- Sharp decreases in weight due to excessive exercising or dieting
- Overuse of steroids
- Conditions such as hormonal imbalances or thyroid disease
Chronic diseases such as liver disease or gastrointestinal disorders
Complications
Compression fractures
The bones that make up your spine (vertebrae) can weaken to the point that they crumple, which may result in back pain, lost height and a hunched posture.
Bone fractures, particularly in the spine or hip, are the most serious complications of osteoporosis. Hip fractures often are caused by a fall and can result in disability and even an increased risk of death within the first year after the injury.
In some cases, spinal fractures can occur even if you haven’t fallen. The bones that make up your spine (vertebrae) can weaken to the point of crumpling, which can result in back pain, lost height and a hunched forward posture.
Sign and Symptom: There typically are no symptoms in the early stages of bone loss. But once your bones have been weakened by osteoporosis, you might have signs and symptoms that include:
- Back pain, caused by a fractured or collapsed vertebra
- Loss of height over time
- A stooped posture
- A bone that breaks much more easily than expected
Diagnosis: The diagnosis of osteoporosis can be made using conventional radiography and by measuring the bone mineral density (BMD).[79] The most popular method of measuring BMD is dual-energy X-ray absorptiometry.
In addition to the detection of abnormal BMD, the diagnosis of osteoporosis requires investigations into potentially modifiable underlying causes; this may be done with blood tests. Depending on the likelihood of an underlying problem, investigations for cancer with metastasis to the bone, multiple myeloma, Cushing’s disease and other above-mentioned causes may be performed.
Conventional radiography[edit]
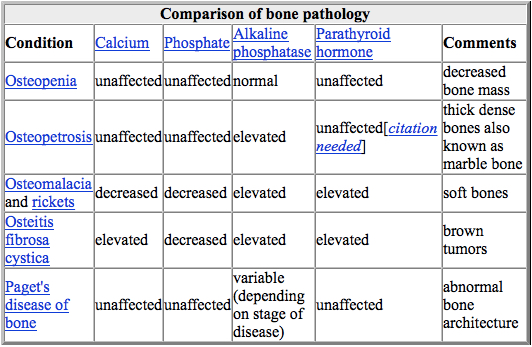
Conventional radiography is useful, both by itself and in conjunction with CT or MRI, for detecting complications of osteopenia (reduced bone mass; pre-osteoporosis), such as fractures; for differential diagnosis of osteopenia; or for follow-up examinations in specific clinical settings, such as soft tissue calcifications, secondary hyperparathyroidism, or osteomalacia in renal osteodystrophy. However, radiography is relatively insensitive to detection of early disease and requires a substantial amount of bone loss (about 30%) to be apparent on X-ray images.
The main radiographic features of generalized osteoporosis are cortical thinning and increased radiolucency. Frequent complications of osteoporosis are vertebral fractures for which spinal radiography can help considerably in diagnosis and follow-up. Vertebral height measurements can objectively be made using plain-film X-rays by using several methods such as height loss together with area reduction, particularly when looking at vertical deformity in T4-L4, or by determining a spinal fracture index that takes into account the number of vertebrae involved. Involvement of multiple vertebral bodies leads to kyphosis of the thoracic spine, leading to what is known as dowager’s hump.
Dual-energy X-ray[edit]
Dual-energy X-ray absorptiometry (DEXA scan) is considered the gold standard for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the bone mineral density is less than or equal to 2.5 standard deviations below that of a young (30–40-year-old[4]:58), healthy adult women reference population. This is translated as a T-score. But because bone density decreases with age, more people become osteoporotic with increasing age.[4]:58 The World Health Organization has established the following diagnostic guidelines:[4][20]
| Category | T-score range | % young women |
| Normal | T-score ≥ −1.0 | 85% |
| Osteopenia | −2.5 < T-score < −1.0 | 14% |
| Osteoporosis | T-score ≤ −2.5 | 0.6% |
| Severe osteoporosis | T-score ≤ −2.5 with fragility fracture[20] |
The International Society for Clinical Densitometry takes the position that a diagnosis of osteoporosis in men under 50 years of age should not be made on the basis of densitometric criteria alone. It also states, for premenopausal women, Z-scores (comparison with age group rather than peak bone mass) rather than T-scores should be used, and the diagnosis of osteoporosis in such women also should not be made on the basis of densitometric criteria alone.[80]
Biomarkers
Chemical biomarkers are a useful tool in detecting bone degradation. The enzyme cathepsin K breaks down type-I collagen, an important constituent in bones. Prepared antibodies can recognize the resulting fragment, called a neoepitope, as a way to diagnose osteoporosis.[81] Increased urinary excretion of C-telopeptides, a type-I collagen breakdown product, also serves as a biomarker for osteoporosis.[82]
Quantitative computed tomography (QCT) differs from DXA in that it gives separate estimates of BMD for trabecular and cortical bone and reports precise volumetric mineral density in mg/cm3 rather than BMD’s relative Z-score. Among QCT’s advantages: it can be performed at axial and peripheral sites, can be calculated from existing CT scans without a separate radiation dose, is sensitive to change over time, can analyze a region of any size or shape, excludes irrelevant tissue such as fat, muscle, and air, and does not require knowledge of the patient’s subpopulation in order to create a clinical score (e.g. the Z-score of all females of a certain age). Among QCT’s disadvantages: it requires a high radiation dose compared to DXA, CT scanners are large and expensive, and because its practice has been less standardized than BMD, its results are more operator-dependent. Peripheral QCT has been introduced to improve upon the limitations of DXA and QCT.[79]
Quantitative ultrasound has many advantages in assessing osteoporosis. The modality is small, no ionizing radiation is involved, measurements can be made quickly and easily, and the cost of the device is low compared with DXA and QCT devices. The calcaneus is the most common skeletal site for quantitative ultrasound assessment because it has a high percentage of trabecular bone that is replaced more often than cortical bone, providing early evidence of metabolic change. Also, the calcaneus is fairly flat and parallel, reducing repositioning errors. The method can be applied to children, neonates, and preterm infants, just as well as to adults.[79] Some ultrasound devices can be used on the tibia
Treatment: Treatment recommendations are often based on an estimate of your risk of breaking a bone in the next 10 years using information such as the bone density test. If your risk isn’t high, treatment might not include medication and might focus instead on modifying risk factors for bone loss and falls.
Biophosphonates
For both men and women at increased risk of fracture, the most widely prescribed osteoporosis medications are bisphosphonates. Examples include:
- Alendronate (Binosto, Fosamax)
- Risedronate (Actonel, Atelvia)
- Ibandronate (Boniva)
- Zoledronic acid (Reclast, Zometa)
Side effects include nausea, abdominal pain and heartburn-like symptoms. These are less likely to occur if the medicine is taken properly.
Intravenous forms of bisphosphonates don’t cause stomach upset but can cause fever, headache and muscle aches for up to three days. It might be easier to schedule a quarterly or yearly injection than to remember to take a weekly or monthly pill, but it can be more costly to do so.
Monoclonal antibody medications
Compared with bisphosphonates, denosumab (Prolia, Xgeva) produces similar or better bone density results and reduces the chance of all types of fractures. Denosumab is delivered via a shot under the skin every six months.
If you take denosumab, you might have to continue to do so indefinitely. Recent research indicates there could be a high risk of spinal column fractures after stopping the drug.
A very rare complication of bisphosphonates and denosumab is a break or crack in the middle of the thighbone.
A second rare complication is delayed healing of the jawbone (osteonecrosis of the jaw). This can occur after an invasive dental procedure such as removing a tooth.
You should have a dental examination before starting these medications, and you should continue to take good care of your teeth and see your dentist regularly while on them. Make sure your dentist knows that you’re taking these medications.
Hormone-related therapy
Estrogen, especially when started soon after menopause, can help maintain bone density. However, estrogen therapy can increase the risk of blood clots, endometrial cancer, breast cancer and possibly heart disease. Therefore, estrogen is typically used for bone health in younger women or in women whose menopausal symptoms also require treatment.
Raloxifene (Evista) mimics estrogen’s beneficial effects on bone density in postmenopausal women, without some of the risks associated with estrogen. Taking this drug can reduce the risk of some types of breast cancer. Hot flashes are a common side effect. Raloxifene also may increase your risk of blood clots.
In men, osteoporosis might be linked with a gradual age-related decline in testosterone levels. Testosterone replacement therapy can help improve symptoms of low testosterone, but osteoporosis medications have been better studied in men to treat osteoporosis and thus are recommended alone or in addition to testosterone.
Bone-building medications
If you can’t tolerate the more common treatments for osteoporosis — or if they don’t work well enough — your doctor might suggest trying:
- Teriparatide (Forteo). This powerful drug is similar to parathyroid hormone and stimulates new bone growth. It’s given by daily injection under the skin. After two years of treatment with teriparatide, another osteoporosis drug is taken to maintain the new bone growth.
- Abaloparatide (Tymlos) is another drug similar to parathyroid hormone. You can take it for only two years, which will be followed by another osteoporosis medication.
- Romosozumab (Evenity). This is the newest bone-building medication to treat osteoporosis. It is given as an injection every month at your doctor’s office. It is limited to one year of treatment, followed by other osteoporosis medications
A new stem cell review
We have talked about how recent progress has been made in treating this disease by removing senescent cells in mice. In this new review, the authors take a look at delivering stem cells to the bone tissue to try to address the imbalance[1].
The idea of increasing the numbers of bone-building stem cells and replacing those lost with age is a plausible approach.
However, this approach is far from complete, as it only addresses one aspect of osteoporosis that causes bones to weaken. Replacing lost stem cells alone is unlikely to solve the problem, as the underlying causes, such as senescent cell accumulation and resulting inflammation, are not being addressed.
Conclusion
As the authors here mention, there are many current stem cell trials, in which researchers are investigating other diseases, that may influence the progression of osteoporosis. This gives us the chance to learn a great deal about stem cell therapies for osteoporosis with some additional effort.
It is plausible that the combination of senescent cell removal therapies and stem cell therapy could be a potent force in treating osteoporosis. Indeed, we have recently seen that reducing chronic age-related inflammation helps to improve stem cell transplants in a recent study.
Prevention
Good nutrition and regular exercise are essential for keeping your bones healthy throughout your life.
Protein
Protein is one of the building blocks of bone. However, there’s conflicting evidence about the impact of protein intake on bone density.
Most people get plenty of protein in their diets, but some do not. Vegetarians and vegans can get enough protein in the diet if they intentionally seek suitable sources, such as soy, nuts, legumes, seeds for vegans and vegetarians, and dairy and eggs for vegetarians.
Older adults might eat less protein for various reasons. If you think you’re not getting enough protein, ask your doctor if supplementation is an option.
Body weight
Being underweight increases the chance of bone loss and fractures. Excess weight is now known to increase the risk of fractures in your arm and wrist. As such, maintaining an appropriate body weight is good for bones just as it is for health in general.
Calcium
Men and women between the ages of 18 and 50 need 1,000 milligrams of calcium a day. This daily amount increases to 1,200 milligrams when women turn 50 and men turn 70.
Good sources of calcium include:
- Low-fat dairy products
- Dark green leafy vegetables
- Canned salmon or sardines with bones
- Soy products, such as tofu
- Calcium-fortified cereals and orange juice
If you find it difficult to get enough calcium from your diet, consider taking calcium supplements. However, too much calcium has been linked to kidney stones. Although yet unclear, some experts suggest that too much calcium especially in supplements can increase the risk of heart disease.
The Health and Medicine Division of the National Academies of Sciences, Engineering and Medicine (formerly the Institute of Medicine) recommends that total calcium intake, from supplements and diet combined, should be no more than 2,000 milligrams daily for people older than 50.
Vitamin D
Vitamin D improves your body’s ability to absorb calcium and improves bone health in other ways. People can get some of their vitamin D from sunlight, but this might not be a good source if you live in a high latitude, if you’re housebound, or if you regularly use sunscreen or avoid the sun because of the risk of skin cancer.
To get enough vitamin D to maintain bone health, it’s recommended that adults ages 51 to 70 get 600 international units (IU) and 800 IU a day after age 70 through food or supplements.
People without other sources of vitamin D and especially with limited sun exposure might need a supplement. Most multivitamin products contain between 600 and 800 IU of vitamin D. Up to 4,000 IU of vitamin D a day is safe for most people.
Exercise
Exercise can help you build strong bones and slow bone loss. Exercise will benefit your bones no matter when you start, but you’ll gain the most benefits if you start exercising regularly when you’re young and continue to exercise throughout your life.
Combine strength training exercises with weight-bearing and balance exercises. Strength training helps strengthen muscles and bones in your arms and upper spine. Weight-bearing exercises — such as walking, jogging, running, stair climbing, skipping rope, skiing and impact-producing sports — affect mainly the bones in your legs, hips and lower spine. Balance exercises such as tai chi can reduce your risk of falling especially as you get older.
Swimming, cycling and exercising on machines such as elliptical trainers can provide a good cardiovascular workout, but they don’t improve bone health.
- Published in Ophthalmology Rehabilitation Center, Services

 English
English  繁體中文
繁體中文  العربية
العربية  Português
Português  Español
Español