Osteoporosis
OSTEOPOROSIS
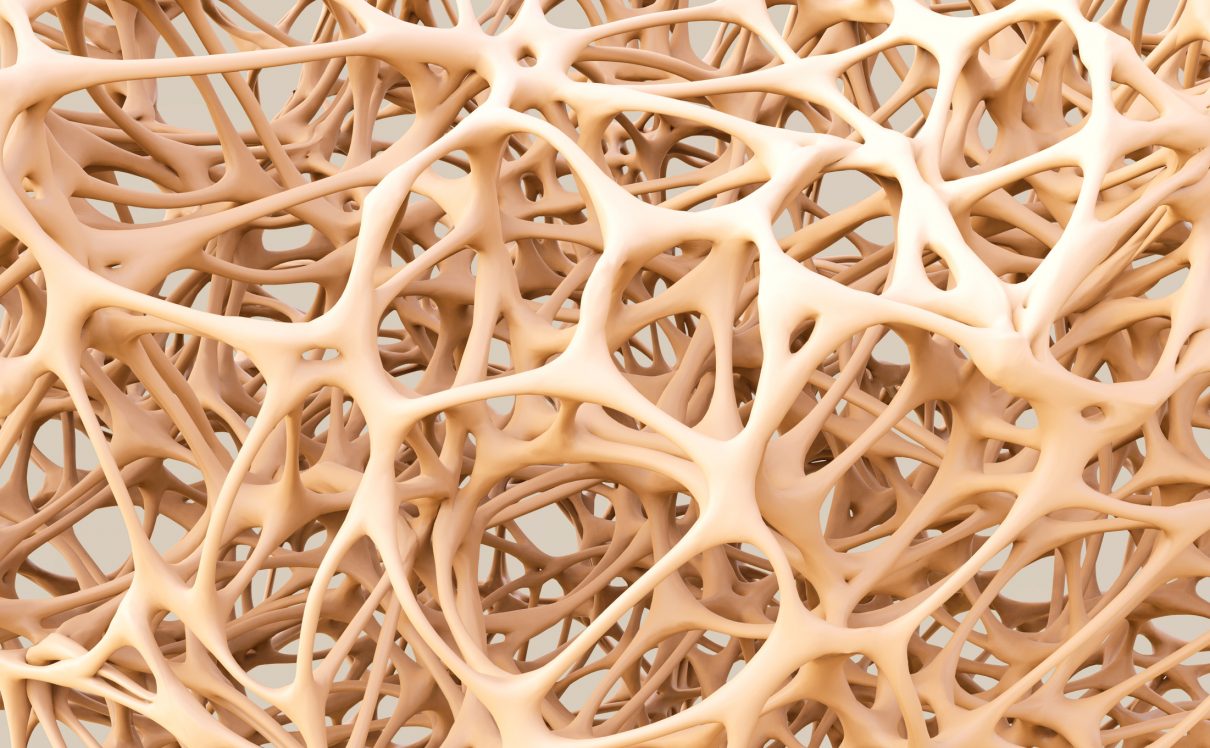
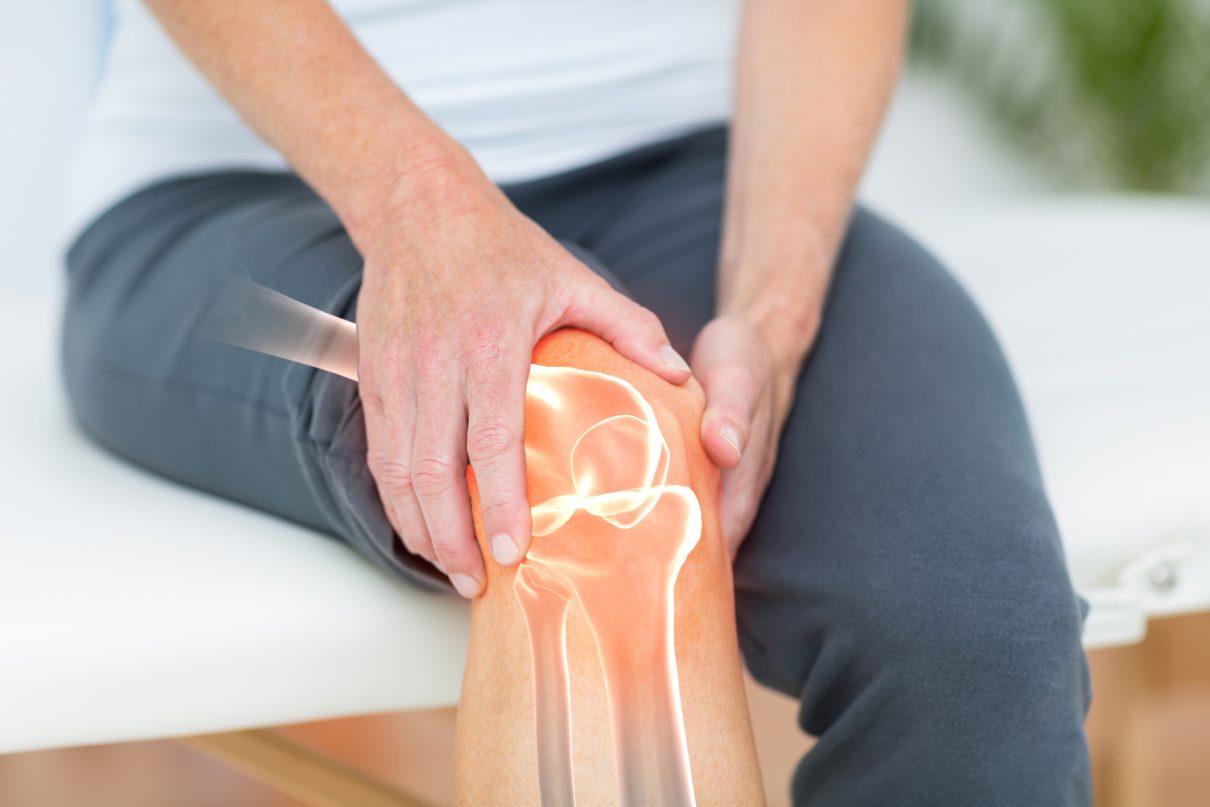
Introduction: Osteoporosis, which literally means porous bone, is a disease in which the density and quality of bone are reduced. As bones become more porous and fragile, the risk of fracture is greatly increased. The loss of bone occurs silently and progressively. Often there are no symptoms until the first fracture occurs.
Osteoporosis affects men and women of all races. But white and Asian women — especially older women who are past menopause — are at highest risk. Medications, healthy diet and weight-bearing exercise can help prevent bone loss or strengthen already weak bones.
As we age some of our bone cells begin to dissolve bone matrix (resorption), while new bone cells deposit osteoid (formation). This process is known as remodeling.
For people with osteoporosis, bone loss outpaces the growth of new bone. Bones become porous, brittle and prone to fracture. Around the world, 1 in 3 women and 1 in 5 men aged fifty years and over are at risk of an osteoporotic fracture. In fact, an osteoporotic fracture is estimated to occur every 3 seconds. The most common fractures associated with osteoporosis occur at the hip, spine and wrist. The likelihood of these fractures occurring, particularly at the hip and spine, increases with age in both women and men.
Of particular concern are vertebral (spinal) and hip fractures. Vertebral fractures can result in serious consequences, including loss of height, intense back pain and deformity (sometimes called Dowager’s Hump). A hip fracture often requires surgery and may result in loss of independence or death.
Cause
- The loss of estrogen due to menopause is the most common cause of osteoporosis. In fact, 25% of women older than 60 years old are found to have osteoporosis.
- Women who go through menopauseearly or those who have had their ovaries surgically removed before the age of 45 are at risk.
The aging process is a major factor because, by the age of 50, bones start thinning by 1-3% every year.
Unchangeable risks
Some risk factors for osteoporosis are out of your control, including:
- Your sex. Women are much more likely to develop osteoporosis than are men.
- Age. The older you get, the greater your risk of osteoporosis.
- Race. You’re at greatest risk of osteoporosis if you’re white or of Asian descent.
- Family history. Having a parent or sibling with osteoporosis puts you at greater risk, especially if your mother or father fractured a hip.
- Body frame size. Men and women who have small body frames tend to have a higher risk because they might have less bone mass to draw from as they age.
Hormone levels
Osteoporosis is more common in people who have too much or too little of certain hormones in their bodies. Examples include:
- Sex hormones. Lowered sex hormone levels tend to weaken bone. The reduction of estrogen levels in women at menopause is one of the strongest risk factors for developing osteoporosis.
Men have a gradual reduction in testosterone levels as they age. Treatments for prostate cancer that reduce testosterone levels in men and treatments for breast cancer that reduce estrogen levels in women are likely to accelerate bone loss.
- Thyroid problems. Too much thyroid hormone can cause bone loss. This can occur if your thyroid is overactive or if you take too much thyroid hormone medication to treat an underactive thyroid.
- Other glands. Osteoporosis has also been associated with overactive parathyroid and adrenal glands.
Dietary factors
Osteoporosis is more likely to occur in people who have:
- Low calcium intake. A lifelong lack of calcium plays a role in the development of osteoporosis. Low calcium intake contributes to diminished bone density, early bone loss and an increased risk of fractures.
- Eating disorders. Severely restricting food intake and being underweight weakens bone in both men and women.
- Gastrointestinal surgery. Surgery to reduce the size of your stomach or to remove part of the intestine limits the amount of surface area available to absorb nutrients, including calcium. These surgeries include those to help you lose weight and for other gastrointestinal disorders.
Steroids and other medications
Long-term use of oral or injected corticosteroid medications, such as prednisone and cortisone, interferes with the bone-rebuilding process. Osteoporosis has also been associated with medications used to combat or prevent:
- Seizures
- Gastric reflux
- Cancer
- Transplant rejection
Medical conditions
The risk of osteoporosis is higher in people who have certain medical problems, including:
- Celiac disease
- Inflammatory bowel disease
- Kidney or liver disease
- Cancer
- Lupus
- Multiple myeloma
- Rheumatoid arthritis
Lifestyle choices
Some bad habits can increase your risk of osteoporosis. Examples include:
- Sedentary lifestyle. People who spend a lot of time sitting have a higher risk of osteoporosis than do those who are more active. Any weight-bearing exercise and activities that promote balance and good posture are beneficial for your bones, but walking, running, jumping, dancing and weightlifting seem particularly helpful.
- Excessive alcohol consumption. Regular consumption of more than two alcoholic drinks a day increases your risk of osteoporosis.
- Tobacco use. The exact role tobacco plays in osteoporosis isn’t clear, but it has been shown that tobacco use contributes to weak bones.
Common risk factors
- A family history of osteoporosis
- European or Asian lineage
- Lack of vitamin D or calcium
- Regular consumption of alcohol or caffeine
- Smoking
- Sharp decreases in weight due to excessive exercising or dieting
- Overuse of steroids
- Conditions such as hormonal imbalances or thyroid disease
Chronic diseases such as liver disease or gastrointestinal disorders
Complications
Compression fractures
The bones that make up your spine (vertebrae) can weaken to the point that they crumple, which may result in back pain, lost height and a hunched posture.
Bone fractures, particularly in the spine or hip, are the most serious complications of osteoporosis. Hip fractures often are caused by a fall and can result in disability and even an increased risk of death within the first year after the injury.
In some cases, spinal fractures can occur even if you haven’t fallen. The bones that make up your spine (vertebrae) can weaken to the point of crumpling, which can result in back pain, lost height and a hunched forward posture.
Sign and Symptom: There typically are no symptoms in the early stages of bone loss. But once your bones have been weakened by osteoporosis, you might have signs and symptoms that include:
- Back pain, caused by a fractured or collapsed vertebra
- Loss of height over time
- A stooped posture
- A bone that breaks much more easily than expected
Diagnosis: The diagnosis of osteoporosis can be made using conventional radiography and by measuring the bone mineral density (BMD).[79] The most popular method of measuring BMD is dual-energy X-ray absorptiometry.
In addition to the detection of abnormal BMD, the diagnosis of osteoporosis requires investigations into potentially modifiable underlying causes; this may be done with blood tests. Depending on the likelihood of an underlying problem, investigations for cancer with metastasis to the bone, multiple myeloma, Cushing’s disease and other above-mentioned causes may be performed.
Conventional radiography[edit]
Conventional radiography is useful, both by itself and in conjunction with CT or MRI, for detecting complications of osteopenia (reduced bone mass; pre-osteoporosis), such as fractures; for differential diagnosis of osteopenia; or for follow-up examinations in specific clinical settings, such as soft tissue calcifications, secondary hyperparathyroidism, or osteomalacia in renal osteodystrophy. However, radiography is relatively insensitive to detection of early disease and requires a substantial amount of bone loss (about 30%) to be apparent on X-ray images.
The main radiographic features of generalized osteoporosis are cortical thinning and increased radiolucency. Frequent complications of osteoporosis are vertebral fractures for which spinal radiography can help considerably in diagnosis and follow-up. Vertebral height measurements can objectively be made using plain-film X-rays by using several methods such as height loss together with area reduction, particularly when looking at vertical deformity in T4-L4, or by determining a spinal fracture index that takes into account the number of vertebrae involved. Involvement of multiple vertebral bodies leads to kyphosis of the thoracic spine, leading to what is known as dowager’s hump.
Dual-energy X-ray[edit]
Dual-energy X-ray absorptiometry (DEXA scan) is considered the gold standard for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the bone mineral density is less than or equal to 2.5 standard deviations below that of a young (30–40-year-old[4]:58), healthy adult women reference population. This is translated as a T-score. But because bone density decreases with age, more people become osteoporotic with increasing age.[4]:58 The World Health Organization has established the following diagnostic guidelines:[4][20]
| Category | T-score range | % young women |
| Normal | T-score ≥ −1.0 | 85% |
| Osteopenia | −2.5 < T-score < −1.0 | 14% |
| Osteoporosis | T-score ≤ −2.5 | 0.6% |
| Severe osteoporosis | T-score ≤ −2.5 with fragility fracture[20] |
The International Society for Clinical Densitometry takes the position that a diagnosis of osteoporosis in men under 50 years of age should not be made on the basis of densitometric criteria alone. It also states, for premenopausal women, Z-scores (comparison with age group rather than peak bone mass) rather than T-scores should be used, and the diagnosis of osteoporosis in such women also should not be made on the basis of densitometric criteria alone.[80]
Biomarkers
Chemical biomarkers are a useful tool in detecting bone degradation. The enzyme cathepsin K breaks down type-I collagen, an important constituent in bones. Prepared antibodies can recognize the resulting fragment, called a neoepitope, as a way to diagnose osteoporosis.[81] Increased urinary excretion of C-telopeptides, a type-I collagen breakdown product, also serves as a biomarker for osteoporosis.[82]
Quantitative computed tomography (QCT) differs from DXA in that it gives separate estimates of BMD for trabecular and cortical bone and reports precise volumetric mineral density in mg/cm3 rather than BMD’s relative Z-score. Among QCT’s advantages: it can be performed at axial and peripheral sites, can be calculated from existing CT scans without a separate radiation dose, is sensitive to change over time, can analyze a region of any size or shape, excludes irrelevant tissue such as fat, muscle, and air, and does not require knowledge of the patient’s subpopulation in order to create a clinical score (e.g. the Z-score of all females of a certain age). Among QCT’s disadvantages: it requires a high radiation dose compared to DXA, CT scanners are large and expensive, and because its practice has been less standardized than BMD, its results are more operator-dependent. Peripheral QCT has been introduced to improve upon the limitations of DXA and QCT.[79]
Quantitative ultrasound has many advantages in assessing osteoporosis. The modality is small, no ionizing radiation is involved, measurements can be made quickly and easily, and the cost of the device is low compared with DXA and QCT devices. The calcaneus is the most common skeletal site for quantitative ultrasound assessment because it has a high percentage of trabecular bone that is replaced more often than cortical bone, providing early evidence of metabolic change. Also, the calcaneus is fairly flat and parallel, reducing repositioning errors. The method can be applied to children, neonates, and preterm infants, just as well as to adults.[79] Some ultrasound devices can be used on the tibia
Treatment: Treatment recommendations are often based on an estimate of your risk of breaking a bone in the next 10 years using information such as the bone density test. If your risk isn’t high, treatment might not include medication and might focus instead on modifying risk factors for bone loss and falls.
Biophosphonates
For both men and women at increased risk of fracture, the most widely prescribed osteoporosis medications are bisphosphonates. Examples include:
- Alendronate (Binosto, Fosamax)
- Risedronate (Actonel, Atelvia)
- Ibandronate (Boniva)
- Zoledronic acid (Reclast, Zometa)
Side effects include nausea, abdominal pain and heartburn-like symptoms. These are less likely to occur if the medicine is taken properly.
Intravenous forms of bisphosphonates don’t cause stomach upset but can cause fever, headache and muscle aches for up to three days. It might be easier to schedule a quarterly or yearly injection than to remember to take a weekly or monthly pill, but it can be more costly to do so.
Monoclonal antibody medications
Compared with bisphosphonates, denosumab (Prolia, Xgeva) produces similar or better bone density results and reduces the chance of all types of fractures. Denosumab is delivered via a shot under the skin every six months.
If you take denosumab, you might have to continue to do so indefinitely. Recent research indicates there could be a high risk of spinal column fractures after stopping the drug.
A very rare complication of bisphosphonates and denosumab is a break or crack in the middle of the thighbone.
A second rare complication is delayed healing of the jawbone (osteonecrosis of the jaw). This can occur after an invasive dental procedure such as removing a tooth.
You should have a dental examination before starting these medications, and you should continue to take good care of your teeth and see your dentist regularly while on them. Make sure your dentist knows that you’re taking these medications.
Hormone-related therapy
Estrogen, especially when started soon after menopause, can help maintain bone density. However, estrogen therapy can increase the risk of blood clots, endometrial cancer, breast cancer and possibly heart disease. Therefore, estrogen is typically used for bone health in younger women or in women whose menopausal symptoms also require treatment.
Raloxifene (Evista) mimics estrogen’s beneficial effects on bone density in postmenopausal women, without some of the risks associated with estrogen. Taking this drug can reduce the risk of some types of breast cancer. Hot flashes are a common side effect. Raloxifene also may increase your risk of blood clots.
In men, osteoporosis might be linked with a gradual age-related decline in testosterone levels. Testosterone replacement therapy can help improve symptoms of low testosterone, but osteoporosis medications have been better studied in men to treat osteoporosis and thus are recommended alone or in addition to testosterone.
Bone-building medications
If you can’t tolerate the more common treatments for osteoporosis — or if they don’t work well enough — your doctor might suggest trying:
- Teriparatide (Forteo). This powerful drug is similar to parathyroid hormone and stimulates new bone growth. It’s given by daily injection under the skin. After two years of treatment with teriparatide, another osteoporosis drug is taken to maintain the new bone growth.
- Abaloparatide (Tymlos) is another drug similar to parathyroid hormone. You can take it for only two years, which will be followed by another osteoporosis medication.
- Romosozumab (Evenity). This is the newest bone-building medication to treat osteoporosis. It is given as an injection every month at your doctor’s office. It is limited to one year of treatment, followed by other osteoporosis medications
A new stem cell review
We have talked about how recent progress has been made in treating this disease by removing senescent cells in mice. In this new review, the authors take a look at delivering stem cells to the bone tissue to try to address the imbalance[1].
The idea of increasing the numbers of bone-building stem cells and replacing those lost with age is a plausible approach.
However, this approach is far from complete, as it only addresses one aspect of osteoporosis that causes bones to weaken. Replacing lost stem cells alone is unlikely to solve the problem, as the underlying causes, such as senescent cell accumulation and resulting inflammation, are not being addressed.
Conclusion
As the authors here mention, there are many current stem cell trials, in which researchers are investigating other diseases, that may influence the progression of osteoporosis. This gives us the chance to learn a great deal about stem cell therapies for osteoporosis with some additional effort.
It is plausible that the combination of senescent cell removal therapies and stem cell therapy could be a potent force in treating osteoporosis. Indeed, we have recently seen that reducing chronic age-related inflammation helps to improve stem cell transplants in a recent study.
Prevention
Good nutrition and regular exercise are essential for keeping your bones healthy throughout your life.
Protein
Protein is one of the building blocks of bone. However, there’s conflicting evidence about the impact of protein intake on bone density.
Most people get plenty of protein in their diets, but some do not. Vegetarians and vegans can get enough protein in the diet if they intentionally seek suitable sources, such as soy, nuts, legumes, seeds for vegans and vegetarians, and dairy and eggs for vegetarians.
Older adults might eat less protein for various reasons. If you think you’re not getting enough protein, ask your doctor if supplementation is an option.
Body weight
Being underweight increases the chance of bone loss and fractures. Excess weight is now known to increase the risk of fractures in your arm and wrist. As such, maintaining an appropriate body weight is good for bones just as it is for health in general.
Calcium
Men and women between the ages of 18 and 50 need 1,000 milligrams of calcium a day. This daily amount increases to 1,200 milligrams when women turn 50 and men turn 70.
Good sources of calcium include:
- Low-fat dairy products
- Dark green leafy vegetables
- Canned salmon or sardines with bones
- Soy products, such as tofu
- Calcium-fortified cereals and orange juice
If you find it difficult to get enough calcium from your diet, consider taking calcium supplements. However, too much calcium has been linked to kidney stones. Although yet unclear, some experts suggest that too much calcium especially in supplements can increase the risk of heart disease.
The Health and Medicine Division of the National Academies of Sciences, Engineering and Medicine (formerly the Institute of Medicine) recommends that total calcium intake, from supplements and diet combined, should be no more than 2,000 milligrams daily for people older than 50.
Vitamin D
Vitamin D improves your body’s ability to absorb calcium and improves bone health in other ways. People can get some of their vitamin D from sunlight, but this might not be a good source if you live in a high latitude, if you’re housebound, or if you regularly use sunscreen or avoid the sun because of the risk of skin cancer.
To get enough vitamin D to maintain bone health, it’s recommended that adults ages 51 to 70 get 600 international units (IU) and 800 IU a day after age 70 through food or supplements.
People without other sources of vitamin D and especially with limited sun exposure might need a supplement. Most multivitamin products contain between 600 and 800 IU of vitamin D. Up to 4,000 IU of vitamin D a day is safe for most people.
Exercise
Exercise can help you build strong bones and slow bone loss. Exercise will benefit your bones no matter when you start, but you’ll gain the most benefits if you start exercising regularly when you’re young and continue to exercise throughout your life.
Combine strength training exercises with weight-bearing and balance exercises. Strength training helps strengthen muscles and bones in your arms and upper spine. Weight-bearing exercises — such as walking, jogging, running, stair climbing, skipping rope, skiing and impact-producing sports — affect mainly the bones in your legs, hips and lower spine. Balance exercises such as tai chi can reduce your risk of falling especially as you get older.
Swimming, cycling and exercising on machines such as elliptical trainers can provide a good cardiovascular workout, but they don’t improve bone health.
- Published in Ophthalmology Rehabilitation Center, Services
Muscular Dystrophy
Introduction: Muscular dystrophy is a group of diseases that cause progressive weakness and loss of muscle mass. In muscular dystrophy, abnormal genes (mutations) interfere with the production of proteins needed to form healthy muscle.
There are many different kinds of muscular dystrophy. Symptoms of the most common variety begin in childhood, mostly in boys. Other types don’t surface until adulthood.
There’s no cure for muscular dystrophy. But medications and therapy can help manage symptoms and slow the course of the disease.
Causes
Muscular dystrophy is caused by mutations on the X chromosome. Each version of muscular dystrophy is due to a different set of mutations, but all prevent the body from producing dystrophin. Dystrophin is a protein essential for building and repairing muscles.
Duchenne muscular dystrophy is caused by specific mutations in the gene that encodes the cytoskeletal protein dystrophin. Dystrophin makes up just 0.002 percent of the total proteins in striated muscle, but it is an essential molecule for the general functioning of muscles.
Dystrophin is part of an incredibly complex group of proteins that allow muscles to work correctly. The protein helps anchor various components within muscle cells together and links them all to the sarcolemma – the outer membrane.
If dystrophin is absent or deformed, this process does not work correctly, and disruptions occur in the outer membrane. This weakens the muscles and can also actively damage the muscle cells themselves.
In Duchenne muscular dystrophy, dystrophin is almost totally absent; the less dystrophin that is produced, the worse the symptoms and etiology of the disease. In Becker muscular dystrophy, there is a reduction in the amount or size of the dystrophin protein.
The gene coding for dystrophin is the largest known gene in humans. More than 1,000 mutations in this gene have been identified in Duchenne and Becker muscular dystrophy
Sign and Symptom: The main sign of muscular dystrophy is progressive muscle weakness. Specific signs and symptoms begin at different ages and in different muscle groups, depending on the type of muscular dystrophy.
Duchenne type muscular dystrophy
This is the most common form of muscular dystrophy. Although girls can be carriers and mildly affected, it’s much more common in boys.
About one-third of boys with Duchenne muscular dystrophy (DMD) don’t have a family history of the disease, possibly because the gene involved may be subject to sudden abnormal change (spontaneous mutation).
Signs and symptoms typically appear in early childhood and may include:
- Frequent falls
- Difficulty rising from a lying or sitting up position
- Trouble running and jumping
- Waddling gait
- Large calf muscles
- Walking on the toes
- Muscle pain and stiffness
- Learning disabilities
Becker muscular dystrophy
Signs and symptoms are similar to those of Duchenne muscular dystrophy, but tend to be milder and progress more slowly. Symptoms generally begin in the teens but may not occur until the mid-20s or even later.
Other types of muscular dystrophy
Some types of muscular dystrophy are defined by a specific feature or by where in the body symptoms first begin. Examples include:
Facioscapulohumeral (FSHD). Muscle weakness typically begins in the face and shoulders. The shoulder blades might stick out like wings when a person with FSHD raises his or her arms. Onset usually occurs in the teenage years but may begin in childhood or as late as age 40
- Also known as Steinert’s disease, this form is characterized by an inability to relax muscles at will following contractions. Myotonic muscular dystrophy is the most common form of adult-onset muscular dystrophy. Facial and neck muscles are usually the first to be affected.
- Limb-girdle. Hip and shoulder muscles are usually the first affected. People with this type of muscular dystrophy may have difficulty lifting the front part of the foot and as a result may trip frequently. Onset usually begins in childhood or the teenage years..
- This type affects boys and girls and is apparent at birth or before age 2. Some forms progress slowly and cause only mild disability, while others progress rapidly and cause severe impairment.
Complications
The complications of progressive muscle weakness include:
- Trouble walking. Some people with muscular dystrophy eventually need to use a wheelchair.
- Shortening of muscles or tendons around joints (contractures). Contractures can further limit mobility.
- Breathing problems. Progressive weakness can affect the muscles associated with breathing. People with muscular dystrophy may eventually need to use a breathing assistance device (ventilator), initially at night but possibly also during the day.
- Curved spine (scoliosis). Weakened muscles may be unable to hold the spine straight.
- Heart problems. Muscular dystrophy can reduce the efficiency of the heart muscle.
- Swallowing problems. If the muscles involved with swallowing are affected, nutritional problems and aspiration pneumonia may develop. Feeding tubes may be an option.
Diagnosis: There are a variety of techniques used to definitively diagnose muscular dystrophy:
The genetic mutations involved in muscular dystrophy are well known and can be used to make a diagnosis.
- Enzyme assay: Damaged muscles produce creatine kinase (CK). Elevated levels of CK in the absence of other types of muscle damage could suggest muscular dystrophy.
- Genetic testing: As genetic mutations are known to occur in muscular dystrophy, these changes can be screened for.
- Heart monitoring: Electrocardiography and echocardiograms can detect changes in the musculature of the heart. This is especially useful for the diagnosis of myotonic muscular dystrophy.
- Lung monitoring: Checking lung function can give additional evidence.
- An electrode needle is inserted into the muscle to be tested. Electrical activity is measured as you relax and as you gently tighten the muscle. Changes in the pattern of electrical activity can confirm a muscle disease.
- Genetic testing. Blood samples can be examined for mutations in some of the genes that cause different types of muscular dystrophy.
- Muscle biopsy. A small piece of muscle can be removed through an incision or with a hollow needle. Analysis (biopsy) of the tissue sample can distinguish muscular dystrophies from other muscle diseases.
Treatment:
Drugs
The two most commonly prescribed drugs for muscular dystrophy are:
- Corticosteroids: This type of medication can help increase muscle strength and slow progression, but long-term use can weaken bones and increase weight gain.
Heart medications: If the condition impacts the heart, beta blockers and angiotensin-converting enzyme (ACE) inhibitors may help
Therapy
Several types of therapy and assistive devices can improve the quality and sometimes the length of life in people who have muscular dystrophy. Examples include:
- Range-of-motion and stretching exercises. Muscular dystrophy can restrict the flexibility and mobility of joints. Limbs often draw inward and become fixed in that position. Range-of-motion exercises can help to keep joints as flexible as possible.
- Low-impact aerobic exercise, such as walking and swimming, can help maintain strength, mobility and general health. Some types of strengthening exercises also might be helpful. But it’s important to talk to your doctor first because some types of exercise might be harmful.
- Braces can help keep muscles and tendons stretched and flexible, slowing the progression of contractures. Braces can also aid mobility and function by providing support for weakened muscles.
- Mobility aids. Canes, walkers and wheelchairs can help maintain mobility and independence.
- Breathing assistance. As respiratory muscles weaken, a sleep apnea device may help improve oxygen delivery during the night. Some people with severe muscular dystrophy may need to use a machine that forces air in and out of their lungs (ventilator).
What Makes our Stem Cell Treatment for Muscular Dystrophy Effective?
Since 2005, we have been developing comprehensive protocols regarding stem cell treatment for muscular dystrophy to overcome the limitations of conventional therapies. In our protocols, stem cells are combined with specialized therapies for muscular dystrophy that not only focus on helping the patient to cope with their symptoms, but also treat the direct cause of the symptoms by promoting the healing of the affected muscles. We believe that our comprehensive stem cell treatment for muscular dystrophy gives our patients the best chances of improvements, allowing for a better quality of life. Different types of muscular dystrophy can be considered for treatment, such as Duchenne, Becker, Limb Girdle, Fascio-Scapulo Humeral and more.
What potential improvements after therapy?
The purpose of stem cell treatment for muscular dystrophy is to promote the healing and growth of the affected muscles. Thus, various kinds of improvement are possible after our treatment and our past patients have experienced the following*:
- Enlarged muscle mass
- Improved range of movement
- Increased muscle strength
- Decreased tremor occurrence
- Improved balance
- Decreased stiffness
- Improved development (in children)
- Published in Neurological Rehabilitation Center, Services
Motor Neuron Diseases
MOTOR NEURON DISEASES (MNDs)
What is Motor Neuron Disease?
MNDs are a group of rare neurological disorders that progressively damage parts of the nervous system particularly the motor neurons. Motor Neurons are cells that control vital muscle activity responsible for gripping, speaking, walking, breathing and swallowing. This leads to general muscle weakness and is visible with wasting. A disruption in the signals between motor neurons and the muscles causes the dysfunction or loss of voluntary movement.
MNDs may affect children or adults. The causes of MNDs are not clearly known. It may be inherited, or effects of environmental, toxic, viral or a collection of many factors.
Signs and Symptoms:
There are numerous signs and symptoms and usually depend on the type of MND. Early signs and symptoms include:
- weakness in ankles or legs such as easily tripping or sudden difficulty to climb stairs
- slurred speech which gradually develop into difficulty swallowing some food
- weak hand grip manifested in dropping things or difficulty opening jars or buttoning clothes
- Weakness in shoulders that cause difficulty in lifting objects
- Unexplained muscle cramps and twitches
- weight loss or thinning of muscles on arms and legs
- breathing difficulty and shortness of breath not related to common respiratory disorders
Types of Motor Neuron Diseases
- ALS or Lou Gehrig’s disease – the most common type, affecting neurons in the brain and spinal cord. Effects are manifested on the muscles of the arms, legs, mouth, and respiratory system. A person with ALS may live another 3–5 years on avergae, but with proper supportive care, some have more than 10 years.
- Primary lateral sclerosis – rare form of MND that affects the neurons in the brain and progresses slower than ALS. It can still affect a person’s quality of life but is not fatal.
- Progressive bulbar palsy (PBP) – involves the brain stem. The condition causes frequent choking spells, difficulty speaking, eating, and swallowing.
- Progressive muscular atrophy (PMA) – a rare condition that affects the lower motor neurons in the spinal cord and causes slow but progressive muscle wasting, especially in the arms, legs, and mouth.
- Spinal muscular atrophy (SMA) – is an inherited MND that afflicts children caused by a genetic change known as SMA1. It usually affects the trunk, legs, and arms.
Role of Functional Medicine in MND
Functional Medicine for MND is multi-level assessment which usually starts with testing metabolic status, evaluating imbalances at the cellular level, genetic testing, identifying risk factors of the patient’s susceptibility to this disease and to help find out why the disease has occurred in the first place. By understanding each of the imbalances identified the specialist is able to formulate and educate the patient about the most appropriate and beneficial diet and lifestyle modification. Another purpose is to promote the healing of the brain injury which can manifest in improved motor functions, balance, better coordination, decreased fatigue, increase in muscle tone and strength, and other improvements of symptoms through intensive physiotherapy, Robotics training, possible stem cell treatment and FMT.
- Published in Neurological Rehabilitation Center, Services
Chronic pain
What is Chronic Pain?
Chronic pain is a pain that lasts longer than the normal cause of healing, recurring and sometimes persistent all day long but may differ in severity. Even if the injury or illness has been resolved or healed, or managed with pain medications, chronic pain can still be felt from months to years. It affects a person’s quality of life and disrupts activities of daily living. For long term conditions, people with chronic pain develop low self-esteem, angry, depressed, anxious, and frustrated.
What are causes of Chronic Pain?
There are numerous causes of chronic pain but the main types are grouped as the following:
- Neuropathic (nerve-related) pain– caused by damaged or malfunctioning sensory receptors and neurons in the nervous system. One example is sciatica (pain in the back, hip, and upper thigh related to the sciatic nerve).
- Muscle pain– this pain comes from problems with the skeletal muscles. It can affect areas such as the lower back, hips, legs and feet, neck, shoulders, arms, and trunk of the body. It often occurs after an injury or following repetitive motions.
- Inflammatory pain – causes include arthritis, tissue injury, infection or post-surgical complications.
- Mechanical/compressive pain: causes include fractures, disc degeneration, or compression of tissue by tumors, cysts, or bony structures.
Role of Functional Medicine
Functional Medicine focuses on finding the underlying factors that play a role in the patient’s chronic pain. Individualized treatment/therapeutic plans are created after a comprehensive evaluation of the patient’s clinical imbalances, diet, lifestyle, psychological well-being, and results of Functional Lab tests. After identifying the underlying cause of one’s chronic pain, our specialists can advise an integration of programs that can help the patient achieve relief from symptoms. Nutritional status, proper supplementation, appropriate physiotherapy may be combined with shockwave therapy, G-Factor and other related management that directly addresses the underlying causes.
- Published in Orthopedic Rehabilitation Center, Services
ARTHRITIS
What is Arthritis?
Arthritis is a very common problem of inflammation of the joints. It affects both children and adults but is more common as people get older. There are many causes of arthritis such as injury, abnormal metabolism, genetic makeup, infections, and immune system dysfunction. There are about 100 different types with many related conditions. Osteoarthritis being the most common type. Arthritis occurs when the cartilage (cushioning connective tissue) in our joints become thin and wear away, bone rubs against bone which then causes the symptoms.
Signs and Symptoms
Symptoms include swelling, pain, stiffness and decreased range of motion. Symptoms may come and go and can be mild, moderate or severe. The symptoms could stay the same for years, but may progress or get worse over time. Severe arthritis can result in chronic pain, inability to do daily activities and make it difficult to move around. It may greatly affect one’s quality of life. Conventional treatment and management involves medications, physical therapies, patient education and support, surgeries, and many alternative treatments with an assortment of claims.
Role of Functional Medicine in Arthritis
A functional approach recognizes that the immune system is in charge of both inflammation and anti-inflammation processes. Functional medicine exposes the root cause of the confused immune system and when we treat this, the body can realign the natural immune response. The body has the ability to heal itself and it all starts with an initial examination, comprehensive neurologic and metabolic exam with necessary tests.
Functional medicine treatment plan promotes healing and improvement of cell functions through personalized nutrition plan, certain physiotherapy activities applicable to the patient’s specific condition and promising new treatments such as G-Factor and NAD.
Make an appointment
Please click here to make an appointment with our doctor.
- Published in Orthopedic Rehabilitation Center, Services
Oral presentation on 4th Annual Singapore Rehabilitation conference 2015
- Published in Publication
Amyotrophic Lateral Sclerosis (ALS)
What is ALS?
Also known as Lou Gehrig’s disease, ALS is a very aggressive and progressive degenerative neurologic disease affecting the nerve cells of the brain and the spinal cord. Normally, our motor neurons reach from the brain to the spinal cord and from the spinal cord to the muscles throughout the body. With ALS, these motor neurons degenerate and die which cause the loss of muscle control which eventually results in paralysis and losing the ability to speak, eat and breathe subsequently. In cases with rapid progression, it can be fatal within a year from the onset while milder progression may allow survival for many years.
What are the common signs and symptoms of ALS?
- Progressive weakness in any body part
- Loss of muscle mass and tissue
- Spasticity or continuous contraction of muscles
- Dysarthria or difficulty in speaking
- Dysphagia or difficulty in swallowing
- Frequent muscle cramps
- Random twitching
- Trouble with the respiratory system and breathing normally
Other facts about ALS:
- Cause is unknown. About 90 percent of ALS cases occur without family history
- It takes about one year before a final ALS diagnosis is made
- ALS is difficult to diagnose as there is no single test or screening to establish the diagnosis. Diagnosis requires a thorough clinical examination and series of diagnostic tests while ruling out other diseases that look like ALS
- It is not contagious
- The disease can affect anyone throughout the world regardless of race, ethnic or socioeconomic backgrounds.
- The initial symptoms of ALS can vary in different people and with a slow onset
Role of Functional Medicine in ALS
Functional Medicine aims in determining imbalances in metabolic functions through Functional Lab tests as these are mostly overlooked in the treatment of the disease. Lab results help in determining underlying factors that cause the patient’s symptoms. A personalized patient care plan can then be established with in-house adjunctive therapies and nutritional modification directed at the specific needs of the patient. ALS conditions may greatly benefit with antioxidant therapy, stem cell therapy, and DFPP (Double Filtration Plasmapheresis) among many others.
For more information or free online consultation, please send us inquiry.
- Published in Neurological Rehabilitation Center, Services
Spinal Muscular Atrophy
What is Autologous Stromal Vascular Fraction (SVF) Harvesting?
Autologous Stromal Vascular Fraction (SVF) Harvesting is a procedure in which adipose (fat) tissues are removed from the body usually from the belly or back area via liposuctioning. This can be done under local anesthesia as an out-patient procedure. The removed fat tissues then get separated into several components by a specialized container under close system, processed by enzyme, centrifuged and incubated. One of the most important component we get as a result of the separation process is a collection of cells we refer to as stromal vascular fraction (SVF). SVF is a collective term generally referring to mixture of endothelial and mesenchymal stem cells and various type of immune cells such as T cells, B cells, mast cells and macrophages. (Miltenyi Biotec, 2014)
The newly harvested SVF can be used in many ways depending on the need of the patient. Most commonly, it will be used to infuse back, together with platelet rich plasma (PRP), for rejuvenating purpose. Apart from this, it can be injected into knee joints to promote repairing and regeneration of knee cartilage. (Pham, Tian, Bui, et al, 2013) (Radin & Burr, 1984)
SVF can also be used to culture and grow more number of mesenchymal stem cell (MSCs) in a specialized laboratory. MSCs has extensive application in many type of chronic conditions, ranging from neurodegenerative disorders, such as Parkinson Disease, Alzheimer’s Disease, Multiple sclerosis to stable neurologic conditions , such as brain injury, spinal cord injury, cerebral palsy etc. (Gierdano, Galderisi & Marino, 2007) (Mesples, Majeed, Yun, et al, 2013)
What is the advantage of Autologous Stromal Vascular Fraction (SVF) Harvesting?
The Autologous Stromal Vascular Fraction (SVF) Harvesting procedure can be performed as an out-patient procedure, under local anesthesia and only takes few hours to complete. The procedure is minimally invasive thus require very little recovery time for the patients. And because the cells are “extracted” from the patient himself/ herself there are no compatibility issue involve.
Why mesenchymal stem cells (MSCs)?
There are several types of stem cell in the body but MSCs has the most capacity for multilineage differentiation, meaning they can adapt and change to the type of cells that patient’s body need. According to many prominent research institutions MSCs also showed the most promising result when it came to wound healing and tissue regeneration. Numerous studies were done on the potential healing ability of MSCs on different type of chronic conditions. (Wu, Chen, Scott, et al, 2007)
Why do we use choose to harvest from adipose tissue and not bone marrow or blood?
Whether your adult mesenchymal stem cells come from bone marrow, blood or fat probably does not make a much difference in terms of clinical use and its result. Although some centers claim that bone marrow or blood derived cells are superior to fat derived cells, there is no study evidence existed to prove such claim. (Harvesting MSCs from bone marrow require an invasive, painful, and time consuming procedure, while the number of cells yield is not comparable to fat origin. This also occur with getting the MSCs from blood, which require injection of marrow activating factors while the cell yielding is low. The situation may not be in the best clinical and financial interest of both the clinician and the patient. (Cell Surgical Networks, 2015)
What can we do with your harvested SVF cells?
Here at BBH Hospital we offer two options for the newly harvested SVF usages. First option after the harvesting we can infuse SVF with your PRP (both are obtained from the same procedure the procedure) and then re-inject it back to a desired area, intravenous, intradermal, or intra-articular, as agreed per prior discussion during the initial consultation. The second option is to send SVF off to a specialized laboratory where MSCs progenitor cells get separated and cultured to make more MSCs for future use. The newly made MSCs can also be stored in a special cells bank where cells are kept in stage of stasis extending their lifespan by significant amount of time. Having your cell stored in a cell bank mean that we no longer need to harvest your SVF again to get MSCs. The bank can fetch your cells sample to and re-culture the cell to get new MSCs as much as you needed for as long as you needed.
Why choose BBH Hospital?
At BBH Hospital we use a special designed close system to harvest the adipose tissues. This mean in addition to the standard sterile technique which we practice, there is an extra safety net to guard against any possible infections. Here at BBH Hospital the patient’s safety is always our first priority. Another advantage for choosing us is that our harvesting system require shorter amount of time comparing to many other systems – meaning more fresh harvested SVF and MSCs will be available for the patient. Also all of our physicians are registered, board certified, internationally trained, and very experienced in their specialty area. Our team at BBH Hospital will make sure that you will have a good experience and great result.
Here are the outline of what BBH-ASVF package includes
- One hour consultation with physician to discuss treatment, history, medication, goals, and expectation outcomes.
- Adipose cell harvesting procedure
- Stromal vascular fraction (SVF) isolation
- Platelet-riche plasma (PRP) procedure
- Reinfusion procedure, either intravenous, intradermal or intra-articular
- Nursing care during the stay
- Medication required for the procedures
- Surgical suite and equipment usage
- Constant monitoring during the procedure and while in recovery
Optional package
- SVF banking and MSCs culture
References:
Radin, E.L.& Burr, D. B. (1984). Hypothesis: joints can heal. Electronically retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/6729484
Pham, P. V., Bui, K., Ngo, D.Q., et al. (2013). Transplantation of Nonexpanded Adipose Stromal Vascular Fraction and Platelet-Rich Plasma for Articular Cartilage Injury Treatment in Mice Model. Journal of Medical Engineering. Volume 2013 (2013), Article ID 832396, 7 pages
Wu, Y., Chen, L., Scott, P. G., et al. (2007). Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells Journal. Volume 25, Issue 10, pages 2648–2659, October 2007.
Gierdano, A., Galderisi, U. & Marino,I. (2007). From the laboratory bench to the patient’s bedside: An update on clinical trials with mesenchymal stem cells. Journal of Cellular Physiology. Volume 211, Issue 1, pages 27–35, April 2007
Cell Surgical Network. (2015). Why does use liposuction fat rather than bone marrow as a source of stem cells? Electronically retrieved from: http://www.stemcellrevolution.com/about-us/faqs/#11
Miltenyi Biotec. (2014).Preparation of the stromal vascular fraction (SVF) from human lipo-aspirate. Electronically retrieved from: https://www.miltenyibiotec.com/~/media/Files/Navigation/Research/Stem%20Cell/SP_MSC_lipoaspirate_protocol.ashx
Mesples, A., Majeed, N., Yun, Z., et al. (2013). Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus. Med Science Monitor, 2013; 19: 852-857
Corti, S., Nizzardo, M., Nardini, M., et al (2008). Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. Journal of Clinical Investigation, 2008,118: 3330
- Published in Neurological Rehabilitation Center, Services
What is Autism?
Autism, also called autism spectrum disorder (ASD), is a complicated condition that includes problems with communication and behavior. It can involve a wide range of symptoms and skills. There are many types of Autism, most of which are a combination of genetic and environmental factors. The severity and functionality of individuals with autism can range from highly skilled and able to live independently to severely challenging and requiring support in daily life.
Signs and Symptoms of Autism
Autism can be discovered and diagnosed as early as a year and a half through associated developmental delays. Signs indicative of autism usually appear by age 2 or 3. These are the most common signs and symptoms of Autism:
- A lack of or poor eye contact
- A narrow range of interests or intense interest in certain topics
- Doing something over and over-repeating words or phrases, rocking back and forth
- High sensitivity to sounds, touches, smells, or sights that seem ordinary to other people
- Not looking at or listening to other people or things
- Problems understanding or using speech, gestures, facial expressions, or tone of voice
- Talking in a sing-song, flat, or robotic voice
- Trouble adapting to changes in routine
Several factors may accompany the development of autism, such as sensory sensitivities, gastrointestinal (GI) disorders, seizures, sleep problems and mental health challenges such as anxiety, depression and attention issues.
Role of Functional Medicine in Autism
Functional medicine seeks to determine the symptom picture and recognizes that one factor is the genetic predisposition, or variations in certain genes which lead to production of abnormal proteins. These abnormal proteins affect different physiologic functions, such as the effectiveness of digestive enzymes in gastrointestinal system, or detoxification enzymes in the liver. This can lead to malfunction of the process within the organ which causes other symptoms.
Functional medicine determines what changes can be applicable, particularly to the environmental, diet and lifestyle factors which will enhance gene expression to its best level. Another goal is to counter any metabolic issues to reduce other symptoms as much as possible. FMT aids Autistic patients to restore normal gut function and balance, Hyperbaric Oxygen Therapy, stem cells and other adjuvant treatments that are identified through thorough assessment.

Male Thai 14 years old.
Symptoms
Social interaction problems with a strong aversion to socialising with others. When meeting in a crowd he would avoid eye-contact, would seek sudden escape especially if being touched, and would will push people away immediately. The patient was very afraid of the sound of birds and would have to hold the parents’ hands tightly. He was continuously on medication.
Conventional treatment
Focused on medication to calm down the symptoms and help sleep. He enrolled in courses for Special Needs child periodically.
Functional Medicine Analysis and Diagnosis
On investigation, the following imbalances in the body were found through blood, urine and stool tests.
The ability to remove waste inside the body was under-functioning. The production of organic acids as a function of the liver enzyme is higher than normal.
Antioxidant protection was lower than optimal. As a result the amount of damaged DNA found in the blood test was much higher than acceptable.
Neurotransmitter,s in particular Dopamine. was low. This affects emotional control and the arrangement of thoughts.
Digestion of food was poor with a large amount of incompletely digested food left in the stool. Food intolerance testing found antibodies to proteins from shrimp, crabs, eggs, milk and soy milk.
The 4R Balancing Program
Focused the diet on taking healthy food, vegetables and fruits. The treatment program focused on adding antioxidants, vitamins, and minerals.
A strict diet change was implemented to avoid further challenges to the body from processed foods including food coloring, food preservatives, and heavy metals. Foods with allergenic proteins were removed from the diet.
The patient received treatments in the Hyperbaric Chamber; with 1.5 atomosphere pressure, with 100% oxygen density for 5 days per week to re-establish oxygen perfusion to all parts of the brain.
Specific nutrients were replaced to accelerate the self-repair process. Nutrients that act as methyl donors which are essential for detoxification, amino acids for neurotransmitter production and polyunsaturated fats to reduce inflammation.
Treatment Result
After 3 months significant behavioural improvement was evident. The patient became more comfortable with the sound of birds, and when he heard or saw birds he would avoid them himself. Behavoiour was calmer at school, with an improved ability to socialize and is more generous with others. There was a marked improvement in the ability to follow the teachers’ instructions and involvement with the surrounding environment.
- Published in Services, Special Needs Child Rehabilitation Center
การบรรยายเรื่อง Office Syndrome โดยนักกายภาพบำบัด โรงพยาบาล BBH ที่ Novartis Thailand
คลินิกออฟฟิศซินโดรม โรงพยาบาลบีบีเอช โดยทีมนักกายภาพบำบัด ได้รับเชิญให้บรรยายเรื่องโรคที่เกิดจากการทำงานของพนักงาน Office โดยมีกิจกรรม การให้คำปรึกษา การตรวจอาการเบื้องต้น การแนะนำการจัดท่าทางในการทำงานอย่างถูกต้อง และการทำกายบริหารเพื่อลดความเครียดตึงปวดของกล้ามเนื้อ เพื่อหลีกเลี่ยงและบำบัดอาการเบื้องต้นของโรคออฟฟิศซินโดรม เช่นการปวดกล้ามเนื้อ คอ บ่า ไหล่ และเส้นประสาทต่างๆ ในวันที่ ๒๓ พฤษาคม ๒๕๖๑ ที่สำนักงานใหญ่บริษัท Novartis, The Emquartier.
- Published in News

 ไทย
ไทย  繁體中文
繁體中文  العربية
العربية  Português
Português  Español
Español